Cholette F, Mesa C, Harris A, Ellis H, Cachero K, Lacap P, Galipeau Y, Langlois M-A, Gingras A-C, Yansouni CP, Papenburg J, Cheng MP, Chakraborty P, Stein DR, Caeseele PV, Bartlett S, Krajden M, Goldfarb D, McGeer A, Osiowy C, Hankins C, Mazer B, Drebot M, Kim J, on behalf of the COVID-19 Immunity Task Force (CITF) working group. Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison. PLOS ONE. 2021 Dec 7. doi: 10.1371/journal.pone.0261003
The results and/or conclusions contained in the research do not necessarily reflect the views of all CITF members.
Many Canadian serosurveys have opted to use dried blood spot (DBS) tests as a practical means to study population-level SARS-CoV-2 seroprevalence. Tens of thousands of Canadians have received DBS kits in the mail. In this article, now published in PLOS ONE, researchers, including several CITF members, set out to determine which of the available DBS assays performed the best.
A DBS test can be performed in one’s home by using a spring-loaded lancet to take drops of blood from a finger and place it onto an absorbent filter paper (similar to self-administered glucose tests) (see Figure 1, below). This blood is then used to test for the presence of antibodies against SARS-CoV-2, thereby determining whether an individual has been previously infected with the virus causing COVID-19. Once the DBS card is dry, it is sealed, packaged, and shipped to a lab through regular carrier services. The DBS sample is then processed and can be tested for the presence of SARS-CoV-2 antibodies against different part of the virus.
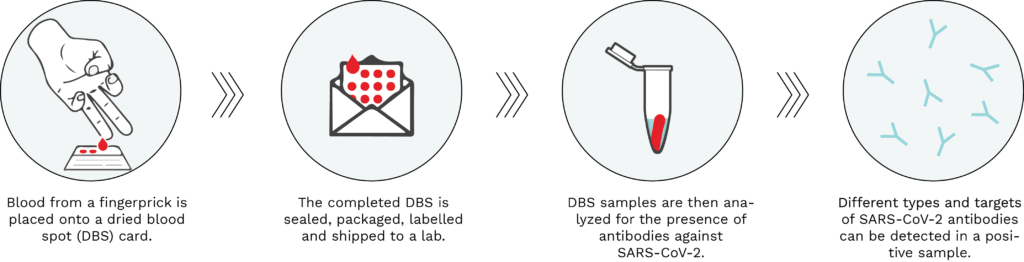
Figure 1: Typical workflow of a dried blood spot serological test. Using a spring-loaded lancet provided in the test kit, drops of blood from a fingerprick are placed onto a filter paper card. The Dried Blood Spot paper is then sealed, packaged, labelled, and shipped to an analytical lab, where it is processed and analyzed for the presence of SARS-CoV-2 antibodies. A positive DBS test result would be indicative of a past infection with the virus causing COVID-19 or vaccination.
Dr. John Kim, Chief of the National Microbiology Laboratory’s HIV Reference Services and CITF Testing and Immune Science Working Group member, set out, with a team of researchers across Canada including many from the CITF, to evaluate a panel of 10 commercially available assays and two made-in-Canada assays engineered to measure SARS-CoV-2 antibodies (see complete list in Table 1 below) to determine which performs best.
To evaluate assay performance, the researchers used 10 blood samples known to be negative for SARS-CoV-2 antibodies and 10 blood samples from individuals who have recovered from COVID-19 and have antibodies to SARS-CoV-2. Next, they measured several test performance indicators. To start, they looked at the ability of the assays to correctly identify the positive samples (i.e., test sensitivity) and negative samples (i.e., test specificity). They also looked at the probability that a positive assay result would accurately represent a true SARS-CoV-2 positive sample (i.e., positive predictive value; PPV) and likewise for the probability of a negative assay result to accurately represent a true SARS-CoV-2 negative sample (i.e., negative predictive value; NPV).
The commercial assays that performed the best, as described by the authors, were the EUROIMMUN IgG spike subunit S1 assay, Roche’s Elecsys quantitative assay detecting total antibodies for the spike protein, and PerkinElmer’s GSP/DELFIA IgG spike subunit S1 assay. All three of these assays identified all positive and negative DBS samples correctly, corresponding to a sensitivity, specificity, PPV and NPV of 100%. Two Made-In-Canada ELISA assays detecting IgG antibodies against SARS-CoV-2’s spike and receptor binding domain (RBD) developed by labs at the University of Ottawa and University of Toronto also achieved a sensitivity, specificity, PPV and NPV of 100%. This evaluation study is of great value to researchers in Canada and abroad looking to select the best assay if they use DBS in their serosurveillance projects.

