Cholette F, Mesa C, Harris A, Ellis H, Cachero K, Lacap P, Galipeau Y, Langlois M-A, Gingras A-C, Yansouni CP, Papenburg J, Cheng MP, Chakraborty P, Stein DR, Caeseele PV, Bartlett S, Krajden M, Goldfarb D, McGeer A, Osiowy C, Hankins C, Mazer B, Drebot M, Kim J, on behalf of the COVID-19 Immunity Task Force (CITF) working group. Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison. Research Square. 2021 Apr 1. doi: 10.21203/rs.3.rs-366992/v1
The results and/or conclusions contained in the research do not necessarily reflect the views of all CITF members.
Many Canadian serosurveys have opted to use dried blood spot (DBS) tests as a practical means to study population-level SARS-CoV-2 prevalence. Tens of thousands of Canadians have received DBS kits in the mail. In this preprint, not yet peer-reviewed, researchers, including several CITF members, set out to determine which of the available DBS assays performed the best. This research was partially funded by the COVID-19 Immunity Task Force (CITF).
A dried blood spot (DBS) test can be performed in the comfort of one’s home by taking drops of blood from a finger using a spring-loaded lancet (similar to self-administered glucose tests) and placing it onto an absorbent filter paper (see Figure 1, below). Blood is used to test for the presence of antibodies against SARS-CoV-2, thereby determining whether an individual has been previously infected with the virus causing COVID-19. Once the DBS card is dry, it is sealed, packaged and shipped to a lab at ambient temperature through regular carrier services. The DBS sample is then processed and tested for the presence of several types and targets of SARS-CoV-2 antibodies.
DBS tests are used by many serosurveys, including many CITF-funded ones, as they provide several advantages over traditional venepuncture (blood draw) collection methods. Foremost, the DBS test kit can be completed at home without needing to travel to a clinic or hospital and avoids taking critical time away from healthcare professionals. For these same reasons, DBS tests are much more accessible to rural and remote communities, an important target of serosurveillance efforts in Canada.
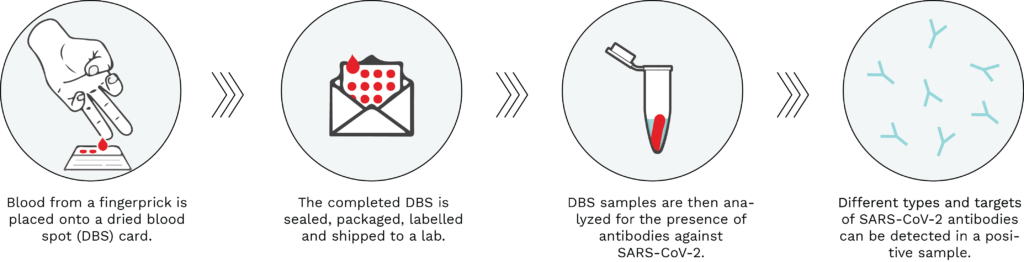
Figure 1: Typical workflow of a dried blood spot serological test. Using a spring-loaded lancet provided in the test kit, drops of blood from a fingerprick are placed onto the DBS filter paper card. The DBS is then sealed, packaged, labelled and shipped to an analytical lab, where it is processed and analyzed for the presence of SARS-CoV-2 antibodies. A positive DBS test result would be indicative of a past infection with the virus causing COVID-19.
While many serosurveys have opted to use DBS-based serological tests, researchers have insofar lacked a comprehensive and comparative review of the different assays available, making choosing which one to use difficult. Dr. John Kim, Chief of the National Microbiology Laboratory’s HIV Reference Services and CITF Testing Working Group member, set out, with a team of researchers across Canada including many from the CITF, to evaluate a panel of 10 commercially available assays and two bespoke assays engineered to measure SARS-CoV-2 antibodies (see complete list in Table 1 below).
To evaluate assay performance, the researchers used 10 blood samples known to be negative for SARS-CoV-2 antibodies and 10 blood samples from individuals who have recovered from COVID-19 and have antibodies to SARS-CoV-2. Next, they measured several test performance indicators. To start, they looked at the ability of the assays to correctly identify the positive samples (i.e., test sensitivity) and negative samples (i.e., test specificity). They also looked at the probability that a positive assay result would accurately represent a true SARS-CoV-2 positive sample (i.e., positive predictive value; PPV) and likewise for the probability of a negative assay result to accurately represent a true SARS-CoV-2 negative sample (i.e., negative predictive value; NPV).
The commercial assays that performed the best, as described by the authors, were the EUROIMMUN IgG spike subunit S1 assay, Roche’s Elecsys quantitative assay detecting total antibodies for the spike protein, and PerkinElmer’s GSP/DELFIA IgG spike subunit S1 assay. All three of these assays identified all positive and negative DBS samples correctly, corresponding to a sensitivity, specificity, PPV and NPV of 100%. Canadian bespoke assays detecting IgG antibodies against SARS-CoV-2’s spike and spike receptor binding domain (RBD) developed by labs at the University of Ottawa and University of Toronto also achieved a sensitivity, specificity, PPV and NPV of 100%. The complete list of test evaluations can be found in the preprint available online, which has not yet gone through the peer-review process. This evaluation study is of great value to researchers in Canada and abroad looking to select the best DBS assay to use in their serosurveillance projects.
Table 1. List of dried blood spot (DBS) assays evaluated, in no particular order.
| Name of Assay | Manufacturer/Developer | |
| 1 | EUROIMMUN | EUROIMMUN, Lübeck, Germany |
| 2 | Platelia | Bio-Rad, Hercules, California |
| 3 | LIASON | DiaSorin, Saluggia, Italy |
| 4 | COV2G | Siemens, Erlangen, Germany |
| 5 | COV2T | Siemens, Erlangen, Germany |
| 6 | Elecsys spike | Roche, Basel, Switzerland |
| 7 | Elecsys nucleocapsid | Roche, Basel, Switzerland |
| 8 | VITROS | Ortho Clinical Diagnostics, Raritan, New Jersey |
| 9 | Architect | Abbott, Mississauga, Canada |
| 10 | GSP/DELFIA | PerkinElmer, Waltham, Massachusetts |
| 11 | In-house spike, RBD and nucleocapsid (U of T) | University of Toronto, Dr. Anne-Claude Gingras |
| 12 | In-house spike, RBD and nucleocapsid (U of O) | University of Ottawa, Dr. Marc-André Langlois |

